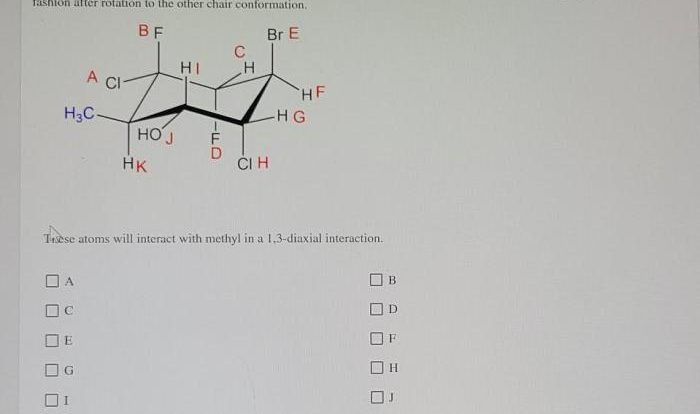
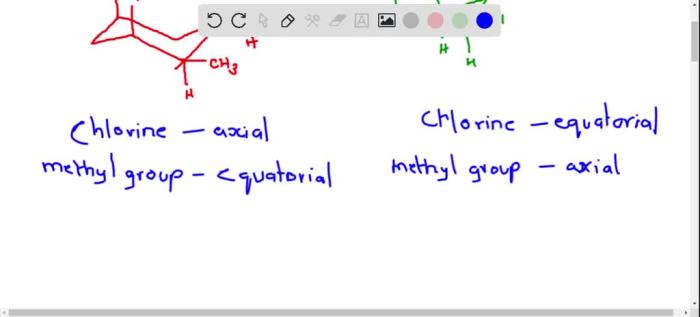
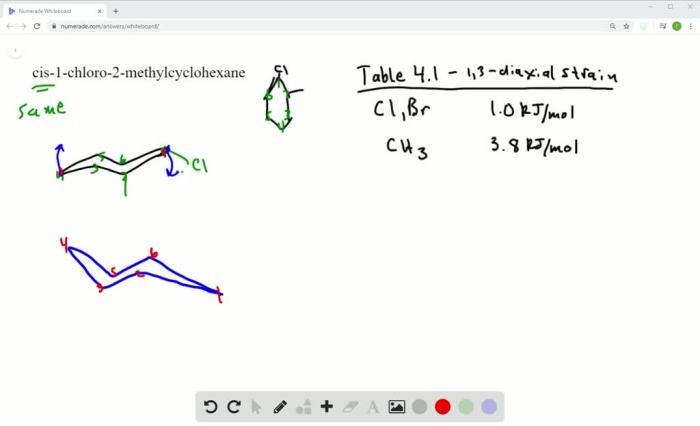
Cis 1 chloro 2 methylcyclohexane – Cis-1-chloro-2-methylcyclohexane, an organic compound with a unique molecular structure, exhibits intriguing properties and finds diverse applications in various fields. This article delves into the synthesis, reactivity, and practical uses of this versatile compound, providing a comprehensive overview of its chemical characteristics and significance.
With its distinct chemical structure, cis-1-chloro-2-methylcyclohexane possesses a six-membered cyclohexane ring substituted with a chlorine atom and a methyl group in specific orientations. This arrangement imparts unique physical and chemical properties to the compound, making it a subject of interest for both fundamental research and practical applications.
Introduction
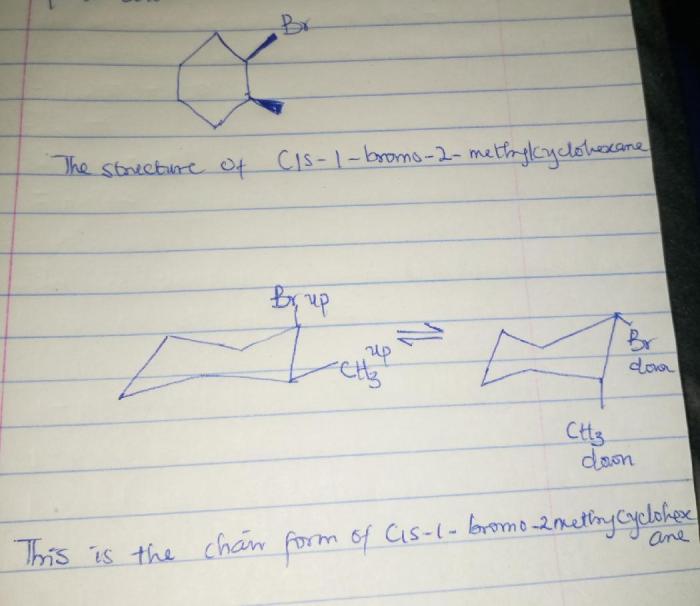
Cis-1-chloro-2-methylcyclohexane is an organic compound belonging to the class of cycloalkanes. It is a colorless liquid with a characteristic odor and is widely used as an intermediate in the synthesis of other organic compounds.
The IUPAC nomenclature of cis-1-chloro-2-methylcyclohexane is 1-chloro-2-methylcyclohexane, indicating the presence of a chlorine atom and a methyl group on the cyclohexane ring. It is also commonly known as 2-chloroisopropylcyclopentane or 1-chloro-2-methylcyclopentane.
Physical and Chemical Properties, Cis 1 chloro 2 methylcyclohexane
- Physical Properties:Cis-1-chloro-2-methylcyclohexane has a molecular weight of 120.62 g/mol, a boiling point of 161-162 °C, and a melting point of -87 °C. It is a colorless liquid with a density of 0.97 g/mL.
- Chemical Properties:Cis-1-chloro-2-methylcyclohexane is a reactive compound due to the presence of the chlorine atom. It undergoes nucleophilic substitution reactions, where the chlorine atom is replaced by another nucleophile. It can also undergo addition reactions, such as hydrogenation and halogenation.
Synthesis: Cis 1 Chloro 2 Methylcyclohexane
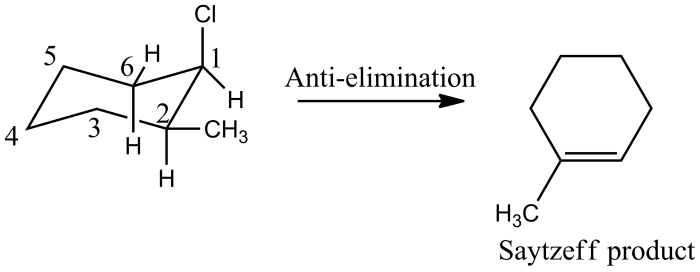
Cis-1-chloro-2-methylcyclohexane can be synthesized through various methods, each involving distinct reaction mechanisms. These methods include:
Hydrochlorination of 1-Methylcyclohexene
In this method, 1-methylcyclohexene reacts with hydrogen chloride (HCl) in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl 3) or zinc chloride (ZnCl 2). The reaction proceeds via an electrophilic addition mechanism, where the electrophile is the protonated HCl molecule.
The proton adds to the double bond, forming a carbocation intermediate, which is then attacked by the chloride ion to yield cis-1-chloro-2-methylcyclohexane.
Reaction Mechanism:
“`
-Methylcyclohexene + HCl + AlCl3→ [1-Methylcyclohexyl] ++ AlCl 4–
[1-Methylcyclohexyl] ++ Cl –→ cis-1-chloro-2-methylcyclohexane“`
Addition of HCl to 1-Methylcyclohexene Oxide
This method involves the reaction of 1-methylcyclohexene oxide with hydrogen chloride. The reaction proceeds via a nucleophilic substitution mechanism, where the nucleophile is the chloride ion. The chloride ion attacks the epoxide ring, opening it and forming cis-1-chloro-2-methylcyclohexane.
Reaction Mechanism:
“`
-Methylcyclohexene oxide + HCl → cis-1-chloro-2-methylcyclohexane
“`
Chlorination of 2-Methylcyclohexanol
In this method, 2-methylcyclohexanol is treated with a chlorinating agent, such as thionyl chloride (SOCl 2) or phosphorus pentachloride (PCl 5). The reaction proceeds via a nucleophilic substitution mechanism, where the nucleophile is the hydroxyl group of the alcohol. The hydroxyl group attacks the chlorinating agent, forming a chloride ion and the desired product, cis-1-chloro-2-methylcyclohexane.
Reaction Mechanism:
“`
-Methylcyclohexanol + SOCl2→ cis-1-chloro-2-methylcyclohexane + SO 2+ HCl
“`
Reactivity
Cis-1-chloro-2-methylcyclohexane is a reactive compound due to the presence of a chlorine atom attached to the cyclohexane ring. This chlorine atom makes the compound susceptible to various nucleophilic and electrophilic reactions.
Nucleophilic Substitution Reactions
In nucleophilic substitution reactions, a nucleophile (an electron-rich species) attacks the electrophilic carbon atom (the carbon atom bonded to the chlorine atom) and replaces the chlorine atom. This reaction results in the formation of a new carbon-nucleophile bond and the breaking of the carbon-chlorine bond.
Electrophilic Addition Reactions
In electrophilic addition reactions, an electrophile (an electron-poor species) adds to the double bond formed by the cyclohexane ring and the chlorine atom. This reaction results in the formation of a new carbon-electrophile bond and the breaking of the chlorine-carbon bond.
Stereochemistry of Reactions
The stereochemistry of these reactions depends on the orientation of the nucleophile or electrophile relative to the cyclohexane ring. In the case of nucleophilic substitution reactions, the nucleophile can attack from either the top or bottom face of the cyclohexane ring, resulting in the formation of either the cis or trans isomer of the product.
In the case of electrophilic addition reactions, the electrophile can add from either the top or bottom face of the cyclohexane ring, resulting in the formation of either the cis or trans isomer of the product.
Applications
Cis-1-chloro-2-methylcyclohexane finds various applications in the chemical industry.
Industrial Uses
- Cis-1-chloro-2-methylcyclohexane is used as a solvent in the production of paints, coatings, and adhesives.
- It is employed in the manufacture of plastics, particularly polyvinyl chloride (PVC).
- It serves as a cleaning agent in the electronics industry.
Applications in Organic Synthesis
- Cis-1-chloro-2-methylcyclohexane is a versatile intermediate in organic synthesis.
- It is used in the preparation of pharmaceuticals, fragrances, and flavors.
- It undergoes reactions such as nucleophilic substitution, elimination, and addition to form various products.
Role as a Solvent or Intermediate
- As a solvent, cis-1-chloro-2-methylcyclohexane offers advantages due to its non-polar nature and ability to dissolve a wide range of organic compounds.
- As an intermediate, it provides a reactive functional group that can be further modified to yield desired products.
Safety Considerations
Cis-1-chloro-2-methylcyclohexane is a flammable and toxic chemical that requires careful handling and storage. Understanding its potential hazards and implementing appropriate safety protocols is crucial to prevent accidents and minimize risks.
The primary hazards associated with cis-1-chloro-2-methylcyclohexane are its:
- Flammability:It is highly flammable and can easily ignite in the presence of heat, sparks, or open flames.
- Toxicity:It is harmful if inhaled, ingested, or absorbed through the skin. Exposure to high concentrations can cause respiratory distress, nausea, vomiting, and even death.
- Irritant:It can cause skin and eye irritation, leading to redness, itching, and discomfort.
Handling and Storage
To ensure safe handling and storage of cis-1-chloro-2-methylcyclohexane, the following protocols should be strictly followed:
- Store in a cool, well-ventilated area away from heat, sparks, and open flames.
- Keep containers tightly sealed when not in use.
- Wear appropriate personal protective equipment (PPE) such as gloves, eye protection, and a respirator when handling.
- Avoid contact with skin and eyes.
- Do not smoke or eat while handling.
Disposal and Environmental Impact
Proper disposal of cis-1-chloro-2-methylcyclohexane is essential to minimize its environmental impact. It should be disposed of in accordance with local regulations and guidelines, typically involving incineration or chemical treatment.
Due to its toxic and flammable nature, uncontrolled release of cis-1-chloro-2-methylcyclohexane into the environment can have detrimental effects on ecosystems and human health. It is therefore crucial to handle and dispose of this chemical responsibly to protect the environment and ensure public safety.
Related Compounds
Cis-1-chloro-2-methylcyclohexane shares structural similarities with other cyclohexane derivatives, such as cyclohexane, methylcyclohexane, and chlorocyclohexane. These compounds all possess a six-membered carbon ring, with varying substituents attached.
The presence of a chlorine atom in cis-1-chloro-2-methylcyclohexane differentiates it from cyclohexane and methylcyclohexane. The chlorine atom’s electronegativity influences the reactivity of the molecule, making it more susceptible to nucleophilic attack compared to the other two compounds.
Impact of Substituents on Reactivity
- Cyclohexane:With no substituents, cyclohexane exhibits relatively low reactivity due to its symmetrical and non-polar nature.
- Methylcyclohexane:The methyl group in methylcyclohexane introduces a slight increase in reactivity compared to cyclohexane, primarily due to the electron-donating properties of the methyl group.
- Chlorocyclohexane:The presence of chlorine in chlorocyclohexane enhances its reactivity significantly. The electronegative chlorine atom withdraws electrons from the ring, creating a partial positive charge that attracts nucleophiles.
- Cis-1-chloro-2-methylcyclohexane:The combination of a chlorine atom and a methyl group in cis-1-chloro-2-methylcyclohexane results in a molecule with intermediate reactivity. The electron-withdrawing effect of chlorine is partially offset by the electron-donating effect of the methyl group.
Spectroscopic Properties
The spectroscopic properties of cis-1-chloro-2-methylcyclohexane provide valuable information about its molecular structure and functional groups.
Infrared (IR) Spectroscopy
The IR spectrum of cis-1-chloro-2-methylcyclohexane exhibits characteristic absorption bands due to the presence of specific functional groups:
- C-H Stretching:A strong band at around 2950-2850 cm -1indicates the presence of C-H bonds in the cyclohexane ring and the methyl group.
- C-Cl Stretching:A strong band at around 700-600 cm -1confirms the presence of the C-Cl bond.
Nuclear Magnetic Resonance (NMR) Spectroscopy
The 1H NMR spectrum of cis-1-chloro-2-methylcyclohexane provides detailed information about the different types of hydrogen atoms present in the molecule:
- Cyclohexane Ring Protons:Two sets of multiplets at around 1.2-1.8 ppm correspond to the axial and equatorial protons on the cyclohexane ring.
- Methyl Protons:A singlet at around 1.0 ppm indicates the presence of the three equivalent protons on the methyl group.
- Methine Proton:A doublet at around 3.5 ppm represents the proton adjacent to the chlorine atom.
Mass Spectrometry (MS)
The mass spectrum of cis-1-chloro-2-methylcyclohexane shows a molecular ion peak at m/z122, corresponding to the molecular formula C 7H 13Cl. Fragmentation patterns can provide further structural information.
FAQ Summary
What are the common methods for synthesizing cis-1-chloro-2-methylcyclohexane?
The most common methods for synthesizing cis-1-chloro-2-methylcyclohexane involve the addition of hydrogen chloride to 1-methylcyclohexene or the reaction of 2-methylcyclohexanol with thionyl chloride.
What are the key reactivity patterns of cis-1-chloro-2-methylcyclohexane?
Cis-1-chloro-2-methylcyclohexane undergoes nucleophilic substitution reactions, primarily via an SN2 mechanism, and electrophilic addition reactions, such as hydrogenation and halogenation.
What are the industrial applications of cis-1-chloro-2-methylcyclohexane?
Cis-1-chloro-2-methylcyclohexane is used as a solvent, an intermediate in the production of other chemicals, and as a starting material for the synthesis of fragrances and flavors.