As a can of soda is placed inside a cooler, a series of physical, chemical, and environmental changes ensue. This article delves into the intricacies of these interactions, providing a comprehensive understanding of the phenomena at play.
The temperature of the soda can undergoes a gradual decrease over time as the cooler’s insulation impedes heat transfer. Condensation forms on the can’s surface due to the temperature difference between the can and the surrounding air, leading to the formation of water droplets.
In certain conditions, frost may accumulate on the can’s surface if the temperature drops below freezing.
A Can of Soda in a Cooler: A Can Of Soda Is Placed Inside A Cooler

Placing a can of soda inside a cooler is a common practice for keeping the beverage cold. This simple act involves various physical, chemical, and environmental processes that can be explored in detail.
Physical Changes
When a can of soda is placed in a cooler, several physical changes occur over time:
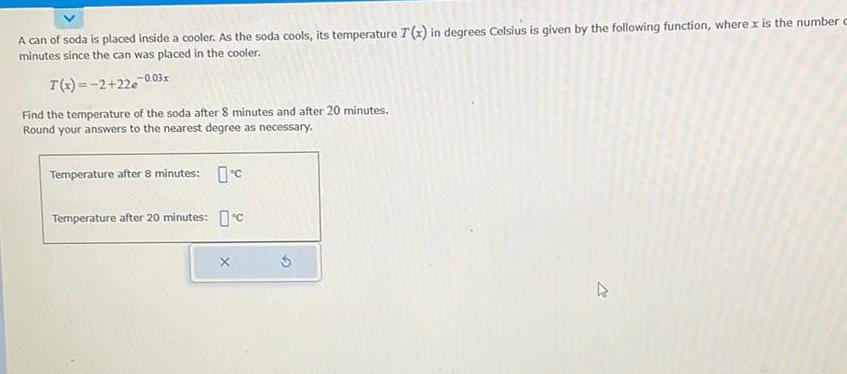
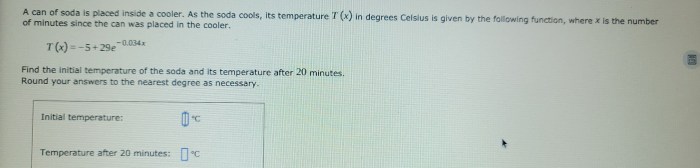
- Temperature Decrease:The temperature of the soda can gradually decreases as it comes into contact with the cooler’s cold environment. The rate of cooling depends on the temperature difference between the can and the cooler, as well as the insulation of the cooler.
- Condensation:As the can cools, water vapor in the air condenses on its surface, forming droplets of water. This process occurs because the cold can lowers the temperature of the surrounding air, causing the water vapor to condense into liquid form.
- Frost Formation:In certain conditions, such as when the cooler is very cold or the can is left inside for an extended period, frost may form on the can’s surface. Frost is formed when water vapor in the air freezes directly into ice crystals.
Chemical Reactions, A can of soda is placed inside a cooler
The presence of a soda can in a cooler can trigger certain chemical reactions, albeit to a limited extent:
- Corrosion:Over time, the acidic contents of the soda can react with the metal of the can, leading to corrosion. This process is accelerated by the presence of moisture, which can form due to condensation on the can’s surface.
- Reaction with Cooler Materials:The materials used in the cooler, such as plastic or metal, may react with the soda’s contents. This can lead to changes in the taste or quality of the beverage, as well as potential leaching of chemicals into the soda.
Energy Transfer
Placing a can of soda in a cooler involves the transfer of energy:
- Convection:Heat is transferred from the soda can to the cooler’s cold environment through convection. The cold air inside the cooler circulates around the can, absorbing heat from it.
- Conduction:Heat is also transferred through conduction, as the cold surfaces of the cooler come into contact with the can’s surface. This process helps to cool the can more efficiently.
- Insulation:The insulation of the cooler plays a crucial role in the rate of energy transfer. Good insulation reduces the amount of heat that can enter or escape the cooler, helping to maintain a cold environment for the soda can.
Environmental Impact
Placing a soda can in a cooler has some environmental implications:
- Waste Disposal:After consuming the soda, the can becomes waste. Proper disposal is essential to prevent littering and ensure recycling. Many communities have recycling programs for aluminum cans, which helps to reduce waste and conserve resources.
- Energy Consumption:Cooling the soda in the cooler requires energy. If the cooler is powered by electricity, it contributes to energy consumption and greenhouse gas emissions. Choosing energy-efficient coolers and using them responsibly can help minimize the environmental impact.
Safety Considerations
There are potential safety hazards associated with placing a soda can in a cooler:
- Pressure Changes:If the cooler is sealed tightly and the soda can is heated, pressure can build up inside the can. This can lead to an explosion or rupture, especially if the can is shaken or dropped. Always handle and store soda cans with caution.
- Spoilage:If the soda is left in the cooler for an extended period, it may spoil. This can occur due to bacterial growth or chemical changes in the beverage. Consuming spoiled soda can lead to health risks.
Creative Applications
Beyond its primary purpose of keeping soda cold, a can of soda placed in a cooler can have various creative applications:
- Cooling Device:The cold soda can can be used as a cooling device for other items. For example, it can be placed in a lunch bag to keep food items cold during a picnic or a day trip.
- Science Experiments:The condensation and frost formation on the can’s surface can be used for educational purposes. They can demonstrate the principles of condensation, evaporation, and heat transfer.
FAQs
What is the primary factor influencing the rate of temperature change in the soda can?
The insulation of the cooler plays a crucial role in determining the rate of temperature change. Better insulation slows down the heat transfer process, leading to a more gradual temperature decrease.
Can chemical reactions occur between the soda and the cooler?
Chemical reactions between the soda and the cooler are unlikely under normal conditions. However, if the cooler is made of certain reactive materials or if the soda is highly acidic, there is a potential for corrosion or other chemical changes.
What environmental concerns should be considered when placing a soda can in a cooler?
The disposal of the soda can and the energy consumption associated with cooling the soda are key environmental considerations. Recycling the can and using energy-efficient coolers can help minimize the environmental impact.