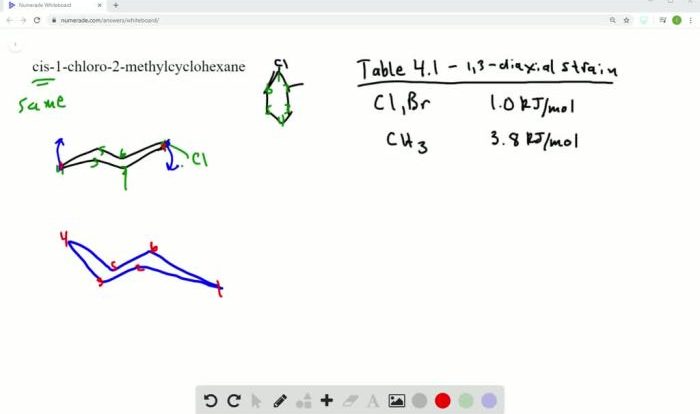
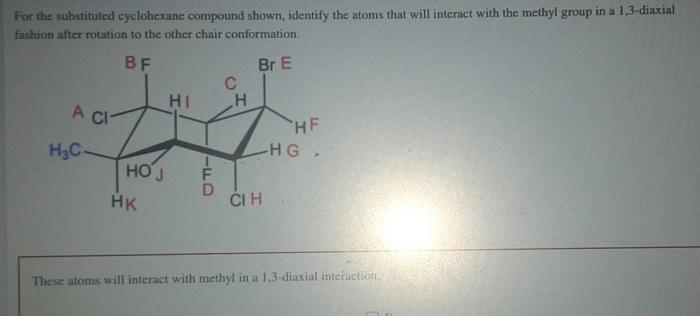
For the substituted cyclohexane compound – Substituted cyclohexane compounds, with their unique structural and chemical properties, play a significant role in various scientific disciplines. This comprehensive guide delves into the intricacies of these compounds, examining their molecular structure, conformational analysis, physical and chemical properties, and diverse applications.
Cyclohexane, a six-membered ring hydrocarbon, forms the foundation of these substituted compounds. The introduction of substituents, such as alkyl groups or functional groups, alters the properties of the cyclohexane ring, giving rise to a wide range of compounds with distinct characteristics.
Cyclohexane Ring Structure
Cyclohexane is a six-carbon cyclic hydrocarbon with the molecular formula C 6H 12. The cyclohexane ring adopts a chair conformation, which is the most stable conformation due to its low steric hindrance and optimal bond angles.
The carbon-carbon bonds in the cyclohexane ring are arranged in a staggered conformation, with each carbon atom bonded to two hydrogen atoms in an axial position and two hydrogen atoms in an equatorial position. The axial hydrogen atoms are oriented perpendicular to the plane of the ring, while the equatorial hydrogen atoms are oriented parallel to the plane of the ring.
Bond Angles, For the substituted cyclohexane compound
The bond angles in the cyclohexane ring are approximately 109.5 degrees, which is close to the ideal tetrahedral bond angle of 109.47 degrees. This indicates that the carbon atoms in the cyclohexane ring are nearly tetrahedral, which contributes to the stability of the chair conformation.
Conformational Flexibility
The cyclohexane ring is conformationally flexible, meaning that it can easily adopt different conformations. The chair conformation is the most stable conformation, but the ring can also adopt boat, twist-boat, and other conformations. The conformational flexibility of the cyclohexane ring is due to the low energy barrier between different conformations.
Substituted Cyclohexane Compounds
Substituted cyclohexane compounds are cyclohexane rings with one or more hydrogen atoms replaced by other atoms or groups of atoms. Substituents can be alkyl groups, halogen atoms, hydroxyl groups, or other functional groups.
Substituents can have a significant impact on the properties of the cyclohexane ring. For example, alkyl substituents can increase the steric hindrance around the ring, which can lead to changes in the conformational preferences of the ring. Halogen substituents can withdraw electrons from the ring, which can make the ring more reactive towards electrophilic attack.
Conformational Analysis: For The Substituted Cyclohexane Compound
Conformational analysis is the study of the different conformations of a molecule and the factors that influence the conformational preferences of the molecule.
Chair Conformation
The chair conformation is the most stable conformation of cyclohexane. In the chair conformation, all of the carbon atoms in the ring are tetrahedral and the hydrogen atoms are arranged in a staggered conformation. The chair conformation has a low steric hindrance and optimal bond angles, which contribute to its stability.
Boat Conformation
The boat conformation is a less stable conformation of cyclohexane. In the boat conformation, two of the carbon atoms in the ring are bent out of the plane of the ring. The boat conformation has a higher steric hindrance than the chair conformation, which makes it less stable.
Twist-Boat Conformation
The twist-boat conformation is a less stable conformation of cyclohexane. In the twist-boat conformation, one of the carbon atoms in the ring is bent out of the plane of the ring and the other carbon atoms are twisted. The twist-boat conformation has a higher steric hindrance than the chair conformation, which makes it less stable.
Factors Influencing Conformational Preferences
The conformational preferences of substituted cyclohexane compounds are influenced by a number of factors, including:
- Steric effects: Steric effects are caused by the interactions between the atoms in a molecule. Steric effects can lead to changes in the conformational preferences of a molecule in order to minimize the steric hindrance between the atoms.
- Electronic effects: Electronic effects are caused by the interactions between the electrons in a molecule. Electronic effects can lead to changes in the conformational preferences of a molecule in order to minimize the energy of the molecule.
Physical and Chemical Properties
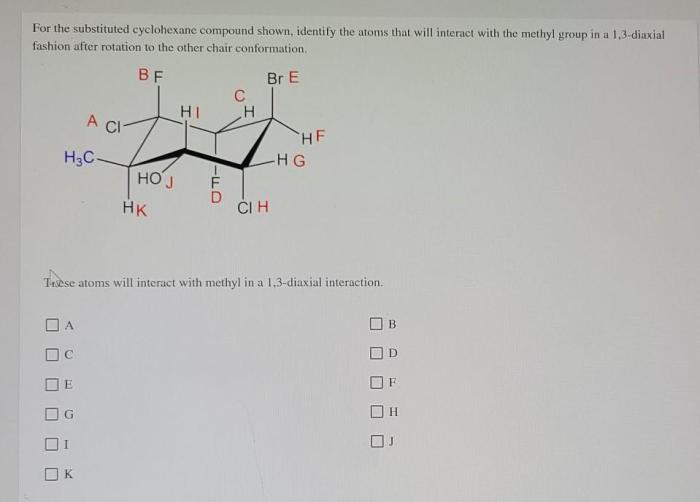
The physical and chemical properties of substituted cyclohexane compounds depend on the nature of the substituents. In general, substituted cyclohexane compounds are colorless liquids or solids with relatively low melting points and boiling points.
The chemical reactivity of substituted cyclohexane compounds depends on the nature of the substituents. Substituents can make the cyclohexane ring more or less reactive towards electrophilic and nucleophilic attack.
Applications
Substituted cyclohexane compounds are used in a wide variety of applications, including:
- Pharmaceuticals: Substituted cyclohexane compounds are used as active ingredients in a variety of pharmaceuticals, including antibiotics, anti-inflammatory drugs, and cholesterol-lowering drugs.
- Materials science: Substituted cyclohexane compounds are used as monomers in the production of polymers, such as nylon and polyethylene.
- Fragrances: Substituted cyclohexane compounds are used as ingredients in fragrances, such as perfumes and colognes.
Commonly Asked Questions
What is the significance of conformational analysis in substituted cyclohexane compounds?
Conformational analysis helps determine the preferred spatial arrangements of substituents on the cyclohexane ring. These arrangements influence the compound’s stability, reactivity, and physical properties.
How do substituents affect the chemical reactivity of cyclohexane compounds?
Substituents can alter the electron density of the cyclohexane ring, making it more or less reactive towards electrophilic or nucleophilic reagents. Electron-withdrawing substituents decrease reactivity, while electron-donating substituents enhance it.