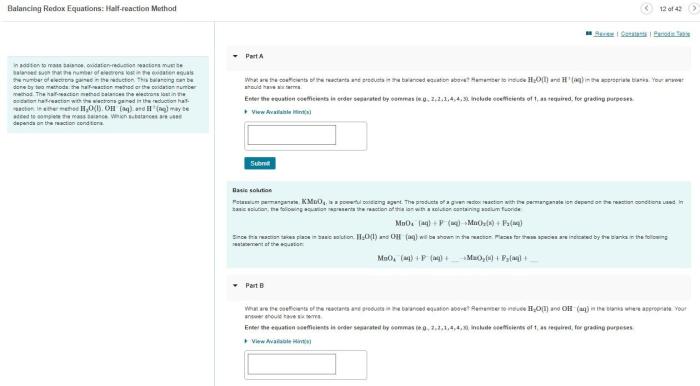
How many formula units are in 5.50 grams of AgNO3? This question delves into the fascinating world of chemical calculations, where we explore the relationship between mass, moles, and formula units. Join us as we embark on a journey to determine the exact number of formula units present in this given mass of silver nitrate.
Silver nitrate, a versatile compound with the chemical formula AgNO3, finds applications in various fields such as photography, jewelry making, and medicine. Understanding the number of formula units present in a specific mass of this compound is crucial for accurate and precise experimentation and analysis.
Overview of Chemical Calculations: How Many Formula Units Are In 5.50 Grams Of Agno3
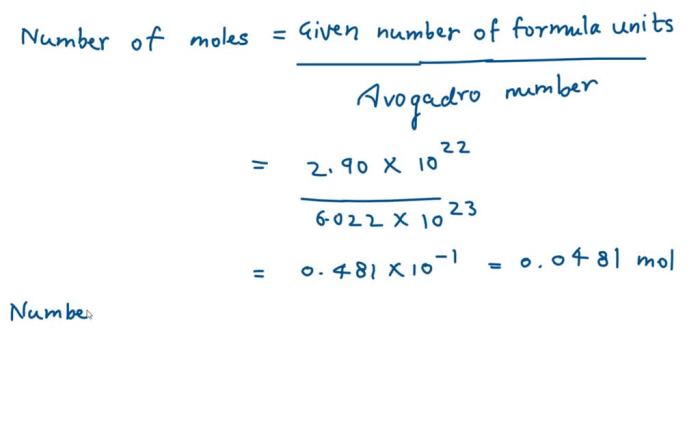
In chemistry, it is often necessary to determine the number of formula units present in a given mass of a compound. This involves using the concept of moles and the relationship between moles and formula units.
The mole is the SI unit of amount of substance. One mole of a substance is defined as the amount of that substance that contains exactly 6.022 × 10 23elementary entities. The elementary entities can be atoms, molecules, ions, or electrons, depending on the substance.
The formula unit is the smallest repeating unit of a compound. For ionic compounds, the formula unit is the simplest whole-number ratio of the ions present in the compound. For molecular compounds, the formula unit is the molecule itself.
The number of formula units in a given mass of a compound can be calculated using the following formula:
Number of formula units = (Mass of compound in grams) / (Molar mass of compound in grams per mole) × (6.022 × 1023formula units per mole)
Determining Molar Mass
The molar mass of a compound is the mass of one mole of that compound. It is calculated by adding the atomic masses of all the atoms in the formula unit.
For example, the molar mass of silver nitrate (AgNO 3) is:
Molar mass of AgNO3= (107.87 g/mol Ag) + (14.01 g/mol N) + (3 × 16.00 g/mol O) = 169.87 g/mol
Converting Mass to Formula Units
To convert a given mass of a compound to the number of formula units, we first convert the mass to grams per mole using the molar mass. Then, we multiply the result by Avogadro’s number (6.022 × 10 23formula units per mole) to obtain the number of formula units.
For example, to convert 5.50 grams of silver nitrate to the number of formula units, we first convert the mass to grams per mole:
Mass of AgNO3in grams per mole = 5.50 g / 169.87 g/mol = 0.0324 mol
Then, we multiply the result by Avogadro’s number:
Number of formula units = 0.0324 mol × 6.022 × 1023formula units per mole = 1.95 × 10 22formula units
Presenting Results, How many formula units are in 5.50 grams of agno3
The calculated number of formula units can be presented in an HTML table as follows:
| Mass of AgNO3 | Number of Formula Units |
|---|---|
| 5.50 grams | 1.95 × 1022 |
Questions Often Asked
What is the molar mass of AgNO3?
The molar mass of AgNO3 is 169.87 g/mol.
How many moles of AgNO3 are present in 5.50 grams?
To calculate the number of moles, we divide the mass by the molar mass: 5.50 g / 169.87 g/mol = 0.0323 moles.
How many formula units are in 0.0323 moles of AgNO3?
Using Avogadro’s number (6.022 x 10^23 formula units/mol), we multiply the number of moles by Avogadro’s number: 0.0323 moles x 6.022 x 10^23 formula units/mol = 1.94 x 10^22 formula units.