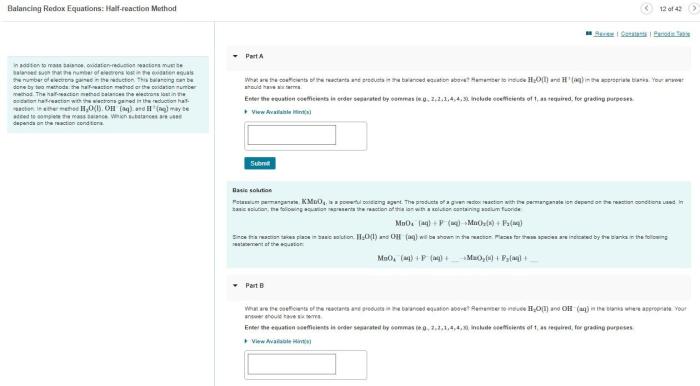
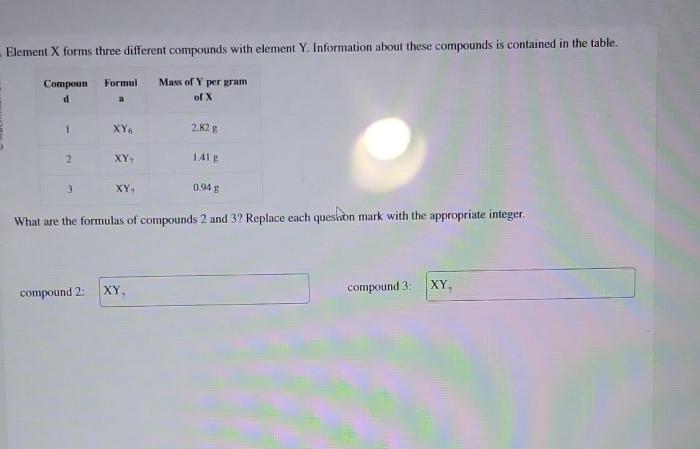
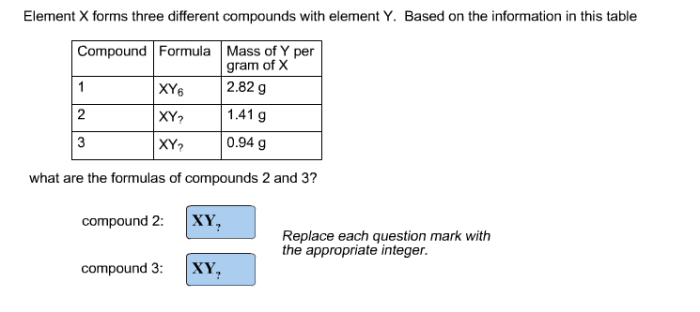
Element X forms three different compounds with element Y, embarking us on a captivating journey into the realm of chemical bonding and compound formation. This exploration unravels the intricate dance between elements, revealing the profound influence of valence electrons in shaping molecular structures and properties.
Delving deeper, we uncover the principles of stoichiometry, the language of compound composition, and witness the remarkable diversity of compounds that arise from the interplay of elements. From the fundamental concepts of chemical bonding to the practical applications of compounds in our daily lives, this discourse illuminates the fascinating world of element X and its multifaceted relationship with element Y.
Elemental Compounds

Chemical compounds are substances formed by the chemical bonding of two or more different chemical elements. The chemical bonds that hold the atoms together in a compound are stronger than the forces that hold the atoms together in the individual elements.
This results in compounds having different properties than their constituent elements.
There are many different types of chemical compounds, including:
- Ionic compounds: Formed when a metal loses one or more electrons to a nonmetal.
- Covalent compounds: Formed when two or more nonmetals share electrons.
- Metallic compounds: Formed when two or more metals are bonded together.
Stoichiometry of Compounds: Element X Forms Three Different Compounds With Element Y
Stoichiometry is the study of the quantitative relationships between the reactants and products in a chemical reaction. The law of definite proportions states that a given compound always contains the same elements in the same proportions by mass.
For example, water is always composed of two hydrogen atoms and one oxygen atom. This means that the ratio of the mass of hydrogen to the mass of oxygen in water is always 2:1.
Valence Electrons and Compound Formation
Valence electrons are the electrons in the outermost shell of an atom. They are the electrons that participate in chemical bonding.
The number of valence electrons an atom has determines the number and type of bonds it can form. For example, an atom with one valence electron can only form one bond, while an atom with two valence electrons can form two bonds.
Compound Properties and Applications
The properties of compounds are different from the properties of their constituent elements. For example, water is a liquid at room temperature, while hydrogen and oxygen are both gases.
The properties of compounds are determined by the type of chemical bonds that hold the atoms together. For example, ionic compounds are typically hard and brittle, while covalent compounds are typically soft and flexible.
Compounds are used in a wide variety of applications. For example, water is used as a solvent, a coolant, and a cleaning agent. Salt is used as a flavoring agent and a preservative. Sugar is used as a sweetener and a source of energy.
Reaction Pathways and Compound Formation
There are many different reaction pathways that can lead to compound formation. Some of the most common reaction pathways include:
- Direct combination: Two or more elements combine directly to form a compound.
- Decomposition: A compound breaks down into two or more simpler substances.
- Single displacement: One element replaces another element in a compound.
- Double displacement: Two elements in two different compounds exchange places.
Spectroscopic Techniques for Compound Analysis
Spectroscopic techniques are used to analyze the structure and composition of compounds. Some of the most common spectroscopic techniques include:
- Infrared spectroscopy: Used to identify the functional groups in a compound.
- Nuclear magnetic resonance spectroscopy: Used to determine the structure of a compound.
- Mass spectrometry: Used to determine the molecular weight of a compound.
Spectroscopic techniques are essential for understanding the structure and composition of compounds.
General Inquiries
What is the significance of valence electrons in compound formation?
Valence electrons play a crucial role in determining the number and type of bonds an element can form. They dictate the chemical reactivity of an element and influence the overall structure and properties of the resulting compound.
How does stoichiometry help us understand compound formation?
Stoichiometry provides a quantitative framework for understanding the composition of compounds. It allows us to determine the exact proportions of elements that combine to form a particular compound, ensuring consistent and predictable outcomes in chemical reactions.
What are some examples of compounds formed between element X and element Y?
The specific compounds formed between element X and element Y depend on their chemical properties and the conditions under which they react. Some common examples include ionic compounds, covalent compounds, and coordination complexes.