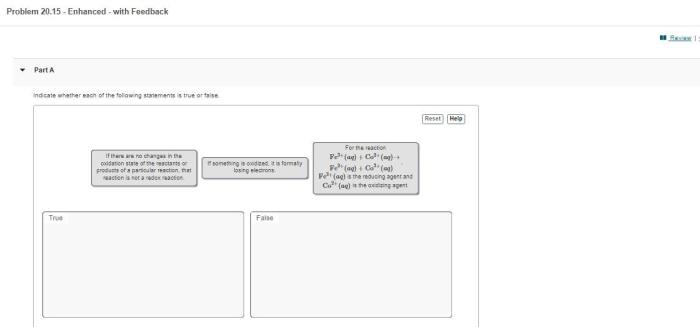
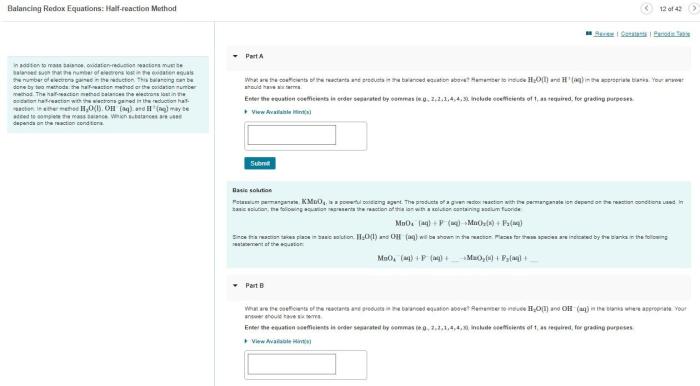
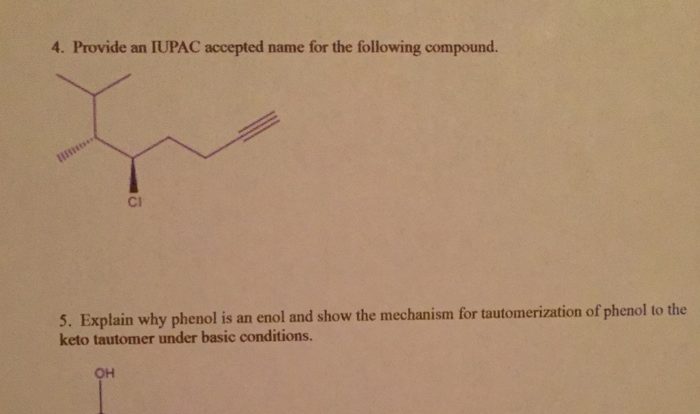
Indicate whether the following balanced equations involve oxidation-reduction. – Oxidation-reduction reactions, also known as redox reactions, are chemical reactions that involve the transfer of electrons between atoms or ions. These reactions play a crucial role in various biological and industrial processes. In this article, we will delve into the concept of oxidation-reduction reactions and provide criteria for identifying them using balanced chemical equations.
Chemical equations represent the stoichiometry of reactions, indicating the relative amounts of reactants and products involved. Balancing these equations ensures that the number of atoms of each element is the same on both sides of the equation. However, balancing equations alone does not provide information about whether a reaction involves oxidation-reduction.
Oxidation-Reduction Reactions: Indicate Whether The Following Balanced Equations Involve Oxidation-reduction.
Oxidation-reduction (redox) reactions involve the transfer of electrons between atoms or ions. In an oxidation reaction, a substance loses electrons, while in a reduction reaction, a substance gains electrons.
The concept of electron transfer is fundamental to understanding redox reactions. Oxidation is defined as the loss of electrons, while reduction is defined as the gain of electrons. In a redox reaction, the oxidizing agent is the substance that accepts electrons and undergoes reduction, while the reducing agent is the substance that donates electrons and undergoes oxidation.
Balancing Chemical Equations, Indicate whether the following balanced equations involve oxidation-reduction.
Chemical equations must be balanced to ensure that the number of atoms of each element is the same on both sides of the equation. Coefficients are used to balance equations and represent the number of molecules or moles of each reactant and product.
Identifying Oxidation-Reduction Reactions
Redox reactions can be identified by several criteria:
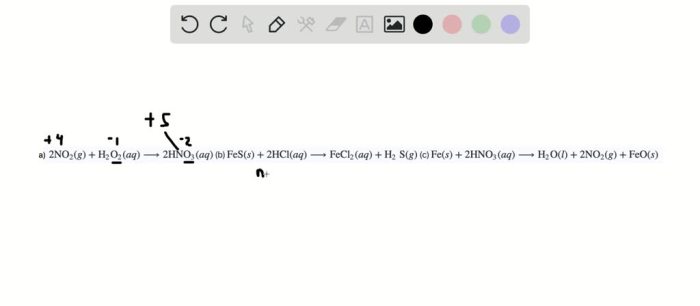
- Change in oxidation number:Oxidation involves an increase in oxidation number, while reduction involves a decrease in oxidation number.
- Presence of oxidizing or reducing agents:Oxidizing agents undergo reduction, while reducing agents undergo oxidation.
- Transfer of electrons:Redox reactions involve the transfer of electrons from one substance to another.
The half-reaction method is a useful tool for identifying redox reactions. In this method, the reaction is divided into two half-reactions, one for oxidation and one for reduction. The half-reactions are then balanced for mass and charge, and the overall redox reaction is obtained by combining the two half-reactions.
Examples
| Balanced Equation | Oxidation-Reduction |
|---|---|
| 2H2 + O2 → 2H2O | Yes |
| 2Na + Cl2 → 2NaCl | Yes |
| Cu + 2AgNO3 → Cu(NO3)2 + 2Ag | Yes |
| CH4 + 2O2 → CO2 + 2H2O | Yes |
| Fe2O3 + 3H2 → 2Fe + 3H2O | Yes |
| NaCl + AgNO3 → No reaction | No |
FAQ Explained
What is the difference between oxidation and reduction?
Oxidation is the loss of electrons, while reduction is the gain of electrons.
How can I identify oxidation-reduction reactions using balanced equations?
Examine the changes in oxidation states of atoms or ions in the reactants and products. If there is a change in oxidation state, the reaction involves oxidation-reduction.
What is the half-reaction method for identifying oxidation-reduction reactions?
The half-reaction method involves separating the reaction into two half-reactions, one for oxidation and one for reduction. Each half-reaction shows the change in oxidation state of the atoms or ions involved.